
Glucagon-like peptide 1 (GLP-1) based therapies have seen a meteoric rise in popularity (about 300%1 increased use between 2018-2023). The first medication in this class (exenatide: Byetta) was FDA approved in 2005 for Type 2 Diabetes Mellitus (DM). Significant weight loss in patients with DM using GLP-1 agonist medications (GLP-1s) was observed. Subsequently, GLP-1s gained FDA approval for weight loss in 2014 (liraglutide: Saxenda/Victoza), 2021 (semaglutide: Wegovy/Ozempic/Rybelsus), and 2023 (tirzepatide: Zepbound/Mounjaro). Semaglutide is also FDA approved for reducing cardiac risk in patients with Type 2 DM.
The common link is that GLP-1s activate receptors that then work on driving down metabolic risk factors through the following mechanisms:
- Enhancing glucose dependent insulin secretion
- Slowing gastric emptying and decreasing appetite
- Reducing postprandial glucagon and food intake
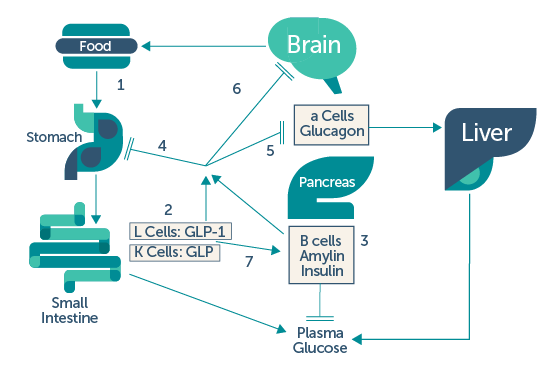
UpToDate2: (1) ingestion of food results in (2) release of gastrointestinal peptides (GLP-1 and GIP: glucose-dependent insulinotropic polypeptide, gastric inhibitory peptide) and (3) pancreatic beta cell hormones (insulin and amylin). GLP-1 and amylin have inhibitory effects on: (4) gastric emptying, (5) glucagon release, and (6) appetite. (7) Following the absorption of food, GLP-1 and GIP promote insulin secretion, otherwise known as the incretin effect.
Use of GLP-1s could also improve excess fat or metabolic associated steatohepatitis (MASH) to prevent advanced liver disease (cirrhosis)3. The choice of GLP-1s can be influenced by co-morbidities.
Potential adverse effects of GLP-1s may include:
- Delayed gastric emptying: Symptoms of delayed gastric emptying may include nausea, vomiting, abdominal pain, or shortness of breath. The American Society of Anesthesiologists (ASA) issued statements (latest October 2024) for procedures involving sedation. Initially, patients were instructed to hold these medications for seven days prior to procedures. More recently, the ASA along with multiple other specialist societies recommended that if patients do continue GLP-1s, they should be on a 24-hour liquid only diet prior to procedure.
- Constipation or Diarrhea
- Pancreatitis: A history of this is a contraindication to starting GLP-1s.
- Personal or family history of medullary thyroid cancer or multiple endocrine neoplasia 2A or 2B.
No conversation about GLP-1s would be complete without addressing access and our cultural messaging around weight. Ideally, the cost of these medications decreases to allow for access across different socioeconomic groups. In our respective medical societies, we may have opportunities to help advocate for better access. There can be many thoughts tied into body weight and shape as a person navigates through their life. As we assist in a patient’s overall health, being mindful on centering the conversation on the health benefits of feeling stronger, more active, and meeting personal health metrics can get us to the goal of connecting the science with the art of medicine.
References
1. Tichy et. al., National trends in prescription drug expenditures and projections for 2022, American Journal of Health-System Pharmacy, 2022.
2. Dungan and DeSantis., Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus. UpToDate, 12.2024.
3. Newsome et al; ESSENCE trial